Cardiovascular (CV) disease is one of the leading causes of death in Canada. It has been estimated that about one in three people in Canada will die from CV disease (many under the age of 65 years), and that it accounts for as many as 32% of all deaths among Canadians.1 In many cases, deaths due to CV disease are preventable. Angiotensin-converting enzyme (ACE) inhibitors have, therefore, become integral parts of the pharmacologic armamentarium to combat this pervasive disease since the introduction of the first such agent, captopril, in the early 1980s. In addition to captopril, the class now includes benazepril, cilazapril, enalapril, fosinopril, lisinopril, perindopril, quinapril, ramipril and trandolapril. All of these agents are available in original brand, and the majority of them are also available in at least one subsequent-entry generic formulation. Most agents can also be found in a fixed-dose combination formulation with a diuretic.2
ACE inhibitors are used for a number of different indications. Broadly, they can be defined as treatment for essential hypertension and the reduction of risk for CV events.2 While each of the ACE inhibitors has a similar set of indications, the weight and quality of clinical trial evidence varies substantially among the individual agents; and not all of them have conclusive proof of benefit for each of the potential indications. The principles of evidence-based medicine dictate that we select treatment agents that have been proven to be effective for the indication for which we are using them. With respect to ACE inhibitors, not only are there major differences between agents in the amount and quality of clinical trial evidence, there are also ample signals from the basic science literature and from trials using surrogate endpoints that suggest many pharmacologic differences among the agents. These differences may have clinical implications.
This review discusses some of these inter-agent differences and provides a brief description of the major clinical trials conducted using ACE inhibitors in a variety of patient populations. Taken together, these two elements highlight the importance of using only proven ACE inhibitors at proven doses.
All ACE inhibitors share the central mechanisms of action of the class—a reduction of the effects of angiotensin II and an increase in the effects of bradykinin. However, there are also many differences between the various agents.
Duration of therapeutic action. Each of the molecules has a distinct pharmacokinetic profile, which includes a wide array of half-lives. Clinically, the differing half-lives help determine the durations of action, which translate into differing profiles of effect (e.g., blood pressure reduction) over time and dictate the number of daily doses that need to be administered to achieve the desired results across the entire 24-hour period. The half-lives of the various ACE inhibitors are shown in Table 1.3 Perindopril and trandolapril have the longest half-lives, while captopril’s is the shortest.
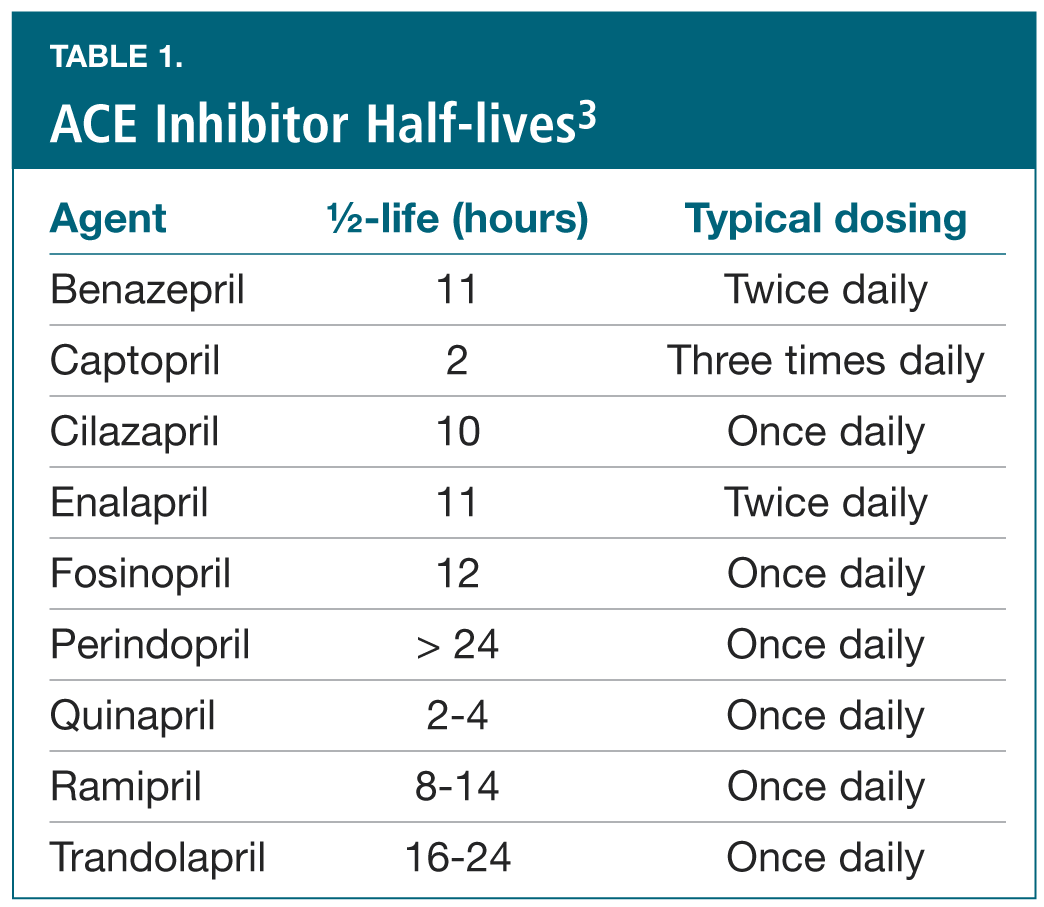
Tissue specificity. ACE inhibitors exert their therapeutic effects through modulation of the renin-angiotensin-aldosterone system (RAAS). This system exists not only in the circulation, but also within organ tissues. It is thought that an important part of the therapeutic activity of ACE inhibitors is their activity within tissues.4 Each of the ACE inhibitors has a different degree of lipophilicity, which influences their ability to penetrate tissues and exert their therapeutic effects locally. Tissue affinity of several ACE inhibitors is shown in Figure 1.5 The highest tissue affinity in this analysis was for perindopril, with captopril having the lowest affinity.
Blood-pressure-independent pleiotropic effects. There have been many studies showing differences between ACE inhibitors with respect to effects on various inflammatory, oxidative, atherogenic, atherothrombotic and profibrinolytic markers. For example, a study compared the pleiotropic effects of enalapril with those of perindopril among 90 normotensive patients with coronary artery disease.6 In that study, treatment with perindopril led to substantially greater reductions in plasma levels of oxidized low-density lipoproteins (LDLs), C-reactive protein (CRP), monocyte chemotactic protein-1 (MCP-1), fibrinogen and plasminogen activator inhibitor-1 (PAI-1), as well as increased interleukin-10 (IL-10), compared to enalapril.
Effects on nitric oxide. Nitric oxide (NO) is a powerful endogenous vasodilator and plays other important beneficial roles in modulating CV risk.7 Increasing its levels or its activity is one of the ways that contribute to the antihypertensive effects of certain medications. All ACE inhibitors enhance the activity of NO, but there is evidence that there are significant differences within the class. In a study examining the effects of several different ACE inhibitors on NO generation in rat aortae, the rank order of potency was perindopril > trandolapril ≈ quinapril ≈ ramipril ≈ enalapril.8
Bradykinin specificity. One of the key mechanisms of therapeutic action of the ACE inhibitors is increase in bradykinin activity. Indeed, some experts argue that this is the primary mechanism of action, as inhibition of angiotensin II formation is only transient with these agents, and the levels of angiotensin II return to baseline over time.9
Bradykinin has been found to be involved in maintenance of normal blood pressure.10 It produces coronary and arterial vasodilation, and improves LV relaxation and contractile performance in congestive heart failure.11 It has also been found to promote myocardial survival following myocardial ischemia.12
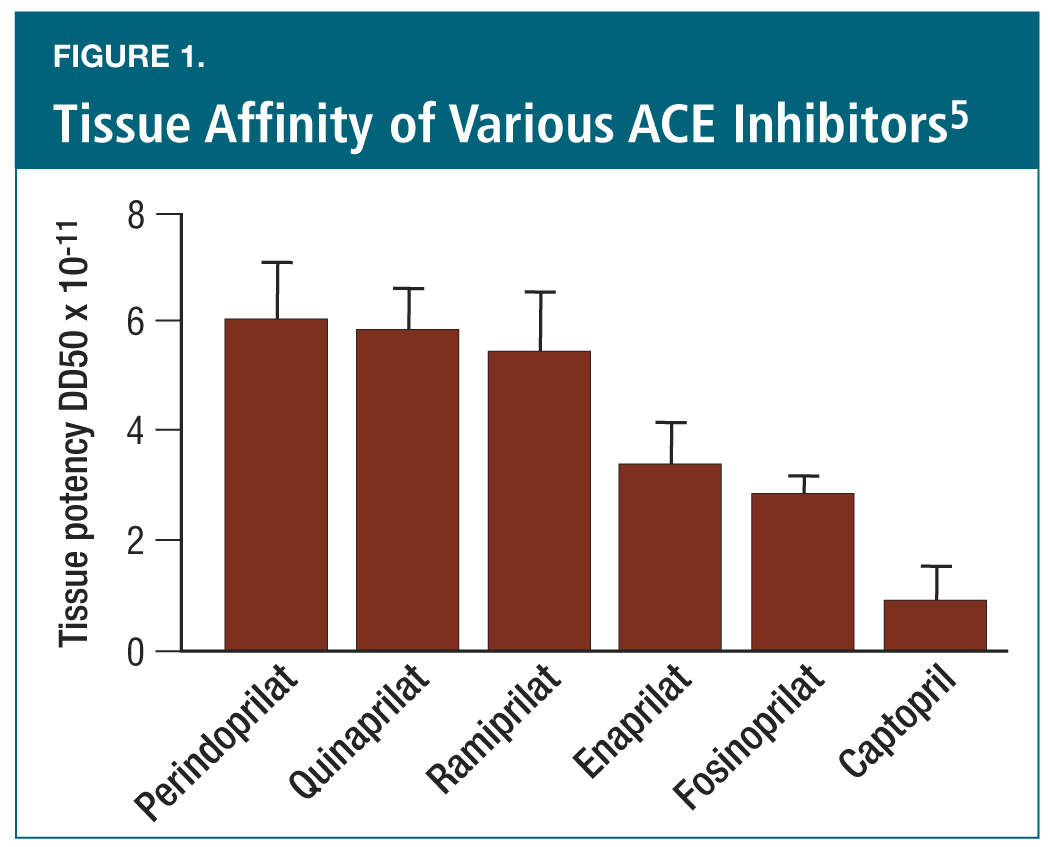
There is evidence showing that the different ACE inhibitors have varying degrees of impact on bradykinin activity. The relative selectivity of the ACE inhibitors for bradykinin sites relative to angiotensin II sites differs significantly between agents, with perindopril having higher selectivity and enalapril having the lowest (Figure 2).13
The current usage of ACE inhibitors for CV risk reduction across the spectrum of CV disease is due to a long track record of beneficial effects in clinical trial in various patient populations.
Heart failure and Post-Myocardial Infarction (MI). The first population in which ACE inhibitors demonstrated significant reductions in CV morbidity and mortality was patients with congestive heart failure. The groundbreaking study in this regard was the CONSENSUS study evaluating enalapril, which showed a 27% reduction in overall mortality over the trial’s average follow-up of 188 days.14 Subsequent to CONSENSUS, several other studies have been published showing significant morbidity and mortality reductions with ACE inhibitors, including studies with perindopril.15,16
Within the post-MI population, the three best known studies are the SAVE study, evaluating captopril for patients post-MI with evidence of left-ventricular dysfunction;17 and the AIRE18 and TRACE19 studies, evaluating ramipril and trandolapril, respectively, among post-MI patients with heart failure. A meta-analysis of these three trials showed that the relative reduction in overall mortality with ACE inhibition in this population was 26%.20 Other ACE inhibitors have also been studied in large post-MI studies, including perindopril, which was associated with a 62% relative risk reduction in the primary composite endpoint of death, hospitalization for heart failure, or left ventricular remodeling among elderly post-infarction patients with preserved left ventricular function (PREAMI study).21 Other agents have shown significant benefits in acute post-MI use, including fosinopril (FAMIS study)22 and lisinopril (GISSI-3 study).23
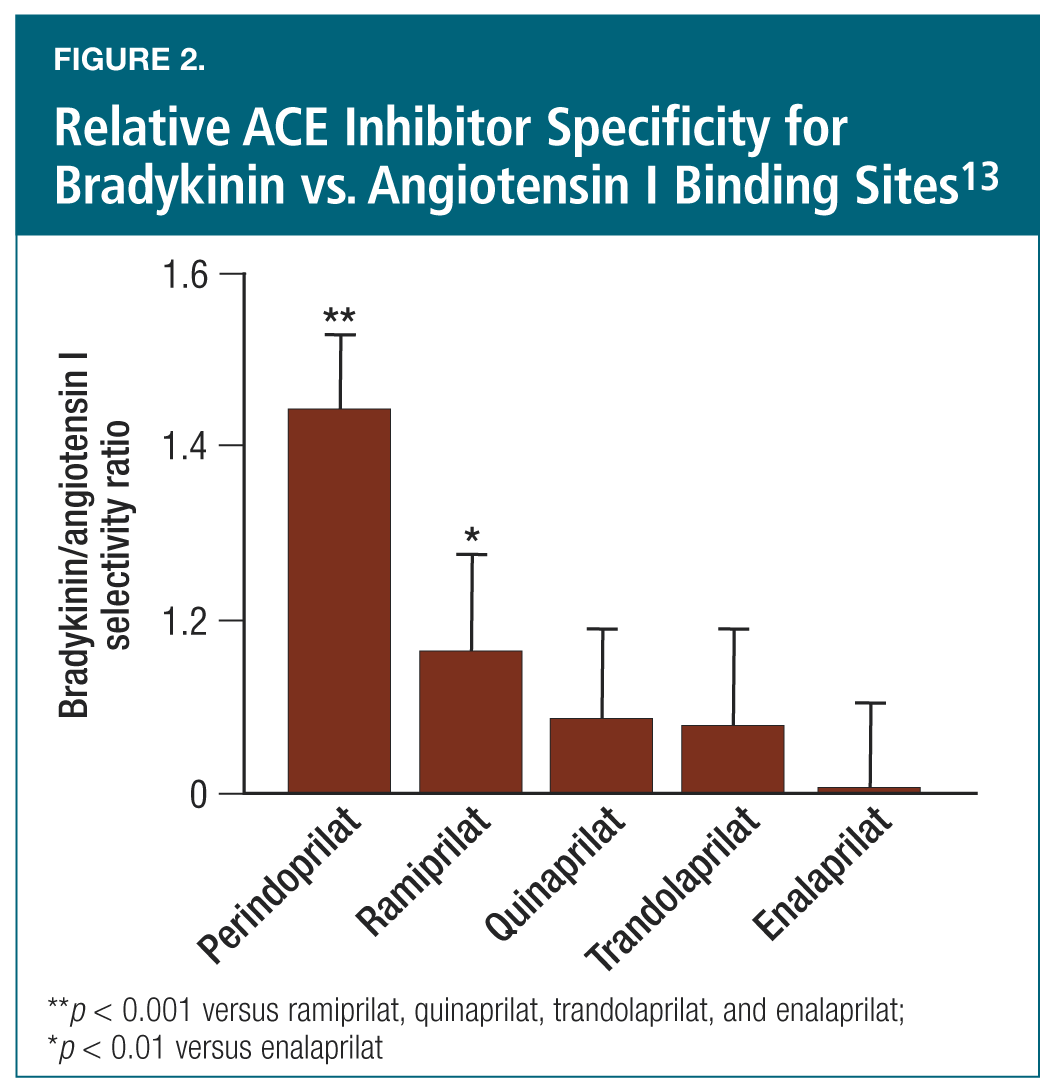
Broader at-risk populations. The use of ACE inhibitors for the reduction of CV risk among a broad spectrum of patients at risk for CV events is largely based on the positive results of two studies in at-risk populations: the HOPE study with ramipril24 and the EUROPA study with perindopril.25 The HOPE study enrolled patients with evidence of vascular disease or diabetes plus one other CV risk factor, and who were not known to have a low ejection fraction or heart failure. EUROPA included patients with stable coronary heart disease and no apparent heart failure. In HOPE, (n = 9,297) ramipril 10 mg daily was associated with a 22% relative risk reduction in major CV events (CV death, nonfatal stroke or nonfatal MI; Figure 3a); while in EUROPA (n = 13,655), the relative risk reduction for perindopril 8 mg daily in the same composite CV endpoint was 20% (Figure 3b).
Another ACE inhibitor that was evaluated in a similar trial was trandolapril in the PEACE study, which evaluated trandolapril 4 mg daily among 8,290 patients with stable coronary artery disease and normal or slightly reduced left ventricular function.26 In this study, there was no significant difference in the primary composite CV endpoint. Also negative was the QUIET study, which evaluated quinapril among patients with coronary artery disease and no evidence of systolic left ventricular dysfunction. In that study, there was no significant benefit of quinapril on the primary composite outcome of ischemic events (the occurrence of cardiac death, resuscitated cardiac arrest, nonfatal MI, coronary artery bypass grafting, coronary angioplasty, or hospitalization for angina pectoris).
ACE inhibitors are crucial components of CV risk reduction for many different patient populations. While all the available agents share their central mechanisms of action (promotion of the benefits of bradykinin and inhibition of the deleterious effects of angiotensin II), there are many differences between the agents that may have clinical implications. These include duration of action, tissue affinity, effects on nitric oxide activity and specificity for bradykinin binding.
Furthermore, evidence-based medicine dictates that we use the agents proven to have beneficial effects and do not assume a class effect. Nor should we assume that a subsequent-entry product would have precisely the same effects as the original tested agent. When prescribing an ACE inhibitor for a particular patient, therefore, the selected agent should be one that has conclusively demonstrated benefit for that type of patient, prescribed at the proven dose.
On the balance of proven clinical trial benefits and mechanistic differences, it appears that perindopril 8 mg daily and ramipril 10 mg daily may be the most useful and versatile of the ACE inhibitors currently available.